Learn More About the Acessa® Procedure

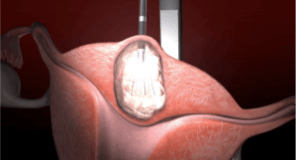
The Acessa procedure is a less invasive treatment option for women with symptomatic uterine fibroids.
The Acessa procedure offers optimized technology for more complete fibroid treatment, provides relief with low reintervention, and allows you to care for more patients by offering more uterine sparing options. Using controlled radiofrequency energy (heat), the Acessa procedure causes coagulative necrosis of the fibroid tissue. The treated tissue softens and shrinks over time, allowing fibroid symptoms to resolve without difficult and time-consuming uterine suturing.1
Clinical Library
Review key publications and clinical data for Lap-RFA with the Acessa ProVu system.
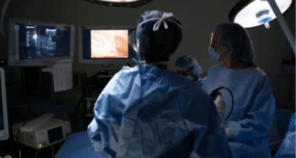
Watch the following videos to see how the Acessa ProVu system has been used in clinical practice.

6 Minute Case Overview

Prior Myomectomy

Advanced Acessa
Case Footage

Resources
Help your patients understand fibroids better with a resource kit that includes social posts, email templates, office posters, and more.
Read the updated ACOG guidelines supporting the use of Lap-RFA – like the Acessa procedure – as a minimally invasive uterine fibroid treatment option.
REFERENCE: 1. Havryliuk Y, Setton R, Carlow JJ, Shaktman BD. Symptomatic Fibroid Management: Systematic Review of the Literature. JSLS. 2017;21(3): e2017.00041. doi:10.4293/JSLS.2017.00041
TRAINING MODULE SOURCES: 1. Acessa ProVu® System, Instructions for Use. — Found in all 6 learning modules. 2. Berman JM, Guido RS, Garza Leal JG, et al. Three-year outcome of the Halt trial: a prospective analysis of radiofrequency volumetric thermal ablation of myomas. J Minim Invasive Gynecol. 2014;21(5):767-774. — Found in Clinical Study Review Module. 3. Brucker SY, Hahn M, Kraemer D, Taran FA, Isaacson KB, Krämer B. Laparoscopic radiofrequency volumetric thermal ablation of fibroids versus laparoscopic myomectomy. Int J Gynaecol Obstet. 2014;125(3):261-265. — Found in Clinical Study Review Module. 4. Chudnoff SG, Berman JM, Levine DJ, Harris M, Guido RS, Banks E. Outpatient procedure for the treatment and relief of symptomatic uterine myomas. Obstet Gynecol. 2013;121(5):1075-1082. — Found in Clinical Study Review Module. 5. Galen DI, Isaacson KB, Lee BB. Does menstrual bleeding decrease after ablation of intramural myomas? A retrospective study. J Minim Invasive Gynecol. 2013;20(6):830-835. — Found in Clinical Study Review Module. 6. Havryliuk Y, Setton R, Carlow JJ, Shaktman BD. Symptomatic Fibroid Management: Systematic Review of the Literature. JSLS. 2017;21(3):e2017.00041. — Found in Clinical Study Review Module. 7. Krämer B, Hahn M, Taran FA, Kraemer D, Isaacson KB, Brucker SY. Interim analysis of a randomized controlled trial comparing laparoscopic radiofrequency volumetric thermal ablation of uterine fibroids with laparoscopic myomectomy. Int J Gynaecol Obstet. 2016;133(2):206-211. — Found in Clinical Study Review Module. 8. Lee BB, Yu SP. Radiofrequency Ablation of Uterine Fibroids: a Review. Curr Obstet Gynecol Rep. 2016;5(4):318-324. — Found in Radiofrequency Ablation and Laparoscopic Ultrasound Module. 9. Levine DJ, Berman JM, Harris M, Chudnoff SG, Whaley FS, Palmer SL. Sensitivity of myoma imaging using laparoscopic ultrasound compared with magnetic resonance imaging and transvaginal ultrasound. J Minim Invasive Gynecol. 2013;20(6):770-774. — Found in Clinical Study Review Module. 10. Leppert PC, Jayes FL, Segars JH. The extracellular matrix contributes to mechanotransduction in uterine fibroids. Obstet Gynecol Int. 2014;2014:783289. — Found in Radiofrequency Ablation and Laparoscopic Ultrasound Module.
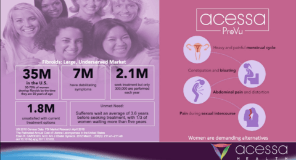
ABOUT FIBROIDS: Fibroids are benign, non-cancerous tumors in a woman’s uterus that often appear during childbearing years. Also called leiomyomas, fibroids are made up of muscle and connective tissue and may range in size from microscopic to larger than a grapefruit. The rate at which they grow is unpredictable, and a woman may have more than one fibroid at a time. While some fibroids are asymptomatic, others can be very painful and cause heavy bleeding, pressure on the bladder or rectum, a distended abdomen, and a lack of energy stemming from anemia. The majority of American women will develop fibroids at some point in their lives. According to the National Institutes of Health (NIH), at least 70 percent of women in the U.S. will develop fibroids by age 50, and the prevalence is even higher among African American women.
This is a general information tool for medical professionals and is not a complete representation of the product Instructions for Use (IFU). It is the medical professional’s responsibility to read and follow the IFU. The information may suggest a particular technique or protocol however it is the sole responsibility of the medical professional to determine which technique or protocol is appropriate. Sound patient evaluation and selection is the responsibility of the medical provider.
IMPORTANT SAFETY INFORMATION
The Acessa ProVu system is indicated for use in percutaneous, laparoscopic coagulation and ablation of soft tissue, including treatment of symptomatic uterine fibroids under laparoscopic ultrasound guidance. The Acessa ProVu system is contraindicated for patients who are not candidates for laparoscopic surgery and/or patients with a uterus adherent to pelvic tissue or viscera. The Acessa ProVu system’s guidance system is not intended for diagnostic use. Please read all instructions for use of the Acessa ProVu system prior to its use. Safe and effective electrosurgery is dependent not only on equipment design but also on factors under control of the operator. Rare but serious risks include, but are not limited to, infection, injury to adjacent structures, blood loss and complications related to laparoscopy and/or general anesthesia. Insufficient data exists on which to evaluate the safety and effectiveness of the Acessa ProVu system in women who plan future pregnancy, therefore the Acessa ProVu system is not recommended for women who are planning future pregnancy.